Collavant n2 - an exclusive native type II collagen ingredient
Joint health
Collavant n2 is an innovative and natural-origin health ingredient recognized as a source of native type II collagen. Collagen plays a role in strengthening cartilage, where it is the main structural protein.
Native type II collagen – also known as undenatured or non-hydrolysed collagen – has a unique mechanism of action : oral tolerance, which can help modulate the immune response against endogenous type II collagen, thereby protecting it from degradation.
According to the studies, Collavant n2 would regulate the inflammatory response, reduce joint discomfort (Manelli et al., 2015) and improve knee function (Bakilan et al., 2016).
Collavant n2 is available in France.
=> This content is intended exclusively for professionals (food supplement laboratories and processors).
Collavant n2 is an innovative health ingredient extracted from chicken sternum cartilage. The manufacturing process of Collavant n2 is standardised, providing a minimum of 25% collagen, including 4% native type II collagen.
In addition to type II collagen, Collavant n2 also contains other compounds such as glycosaminoglycans (GAGs). These macromolecules make up the matrix of connective tissue and ensure the structure and elasticity of most cartilage, skin and tendons.
Given its unique mechanism of action, Collavant n2 helps support joint health.
Native type II collagen: the essential component of cartilage
Type II collagen is the main structural protein in cartilage. Its basic unit is tropocollagen, a glycoprotein composed of three alpha-peptide chains ([α1(II)] 3) bound in a triple-helix quaternary structure 300 nm long (figure 1).
The tropocollagen units bind together to form a collagen fibril, which is roughly 50 nm in diameter. Assembled fibrils form collagen fibers.
Unlike the hydrolyzed collagen composed of short-chain peptide commonly found on the market, native type II collagen contained in Collavant n2 keeps its three-dimensional structure. In its biologically active form, the collagen then preserves its epitopes, the three-dimensional structures that can be recognized by the immune system.
Joint cartilage: role, properties and degradation
Cartilage is a connective tissue covering the ends of bones. It is composed of chondrocytes, collagen and a basic fundamental substance called SFA. This substance is itself composed of proteoglycans and GAGs (chondroitin sulfate and keratan sulfate especially). Cartilage protects and maintains bone structures during movement. The coupled action of collagen and SFA limits bone on bone friction and increases the resistance of joints to high mechanical forces.
In the joints, cartilage is said to be hyaline – in other words, it is full of type II collagen fibers. These are arranged singularly in a special mesh-like network (Thiry et al., 2020). Its composition makes it very elastic and resistant, giving it an important structural role (Shoulders et al., 2009).
Cartilage tissues do not have blood vessels ; its cells receive nutrition by diffusion from the synovial fluid in the joint cavity. For this reason, the natural generation of cartilage is slow and often incomplete.
During physical exercise, or simply because of the daily movements, lesions can appear in the cartilage, fragmenting collagen fibers and disrupting their structure. Cartilage then becomes fragile, losing its resistance and viscoelastic properties.
Moreover, with age, cartilage regeneration becomes slower and slower, and lesions are often irreversible.
Immune system and cartilage degradation
The immune system plays a role in cartilage degradation. Due to the erosion of cartilage or to the presence of lesions, joint cartilage degradation products are released into the extracellular matrix of the joint. The immune system, and more specifically T lymphocytes, do not recognize these endogenous collagen fragments, and therefore categorize them as potentially harmful (Kandahari et al., 2015). Thus, the immune cells, which now consider collagen to be undesirable, will aggravate the fragility of the cartilage and increase inflammation. The inflammatory reaction generates joint pain and can cause an unusually high secretion of synovial fluid, resulting in an effusion.
Many people find themselves confronted with joint pain like this. This is why Elementa offers Collavant n2, an effective solution for promoting joint health with its mechanism of action that acts on the immune-mediated aspect of joint conditions.
Mechanism of action: oral tolerance
Collavant n2 owes its effect to the native type II collagen it contains, through a mechanism of immune mediation called oral tolerance.
This mechanism of action involves administering an antigen that can modulate the immune response as a positive reaction.
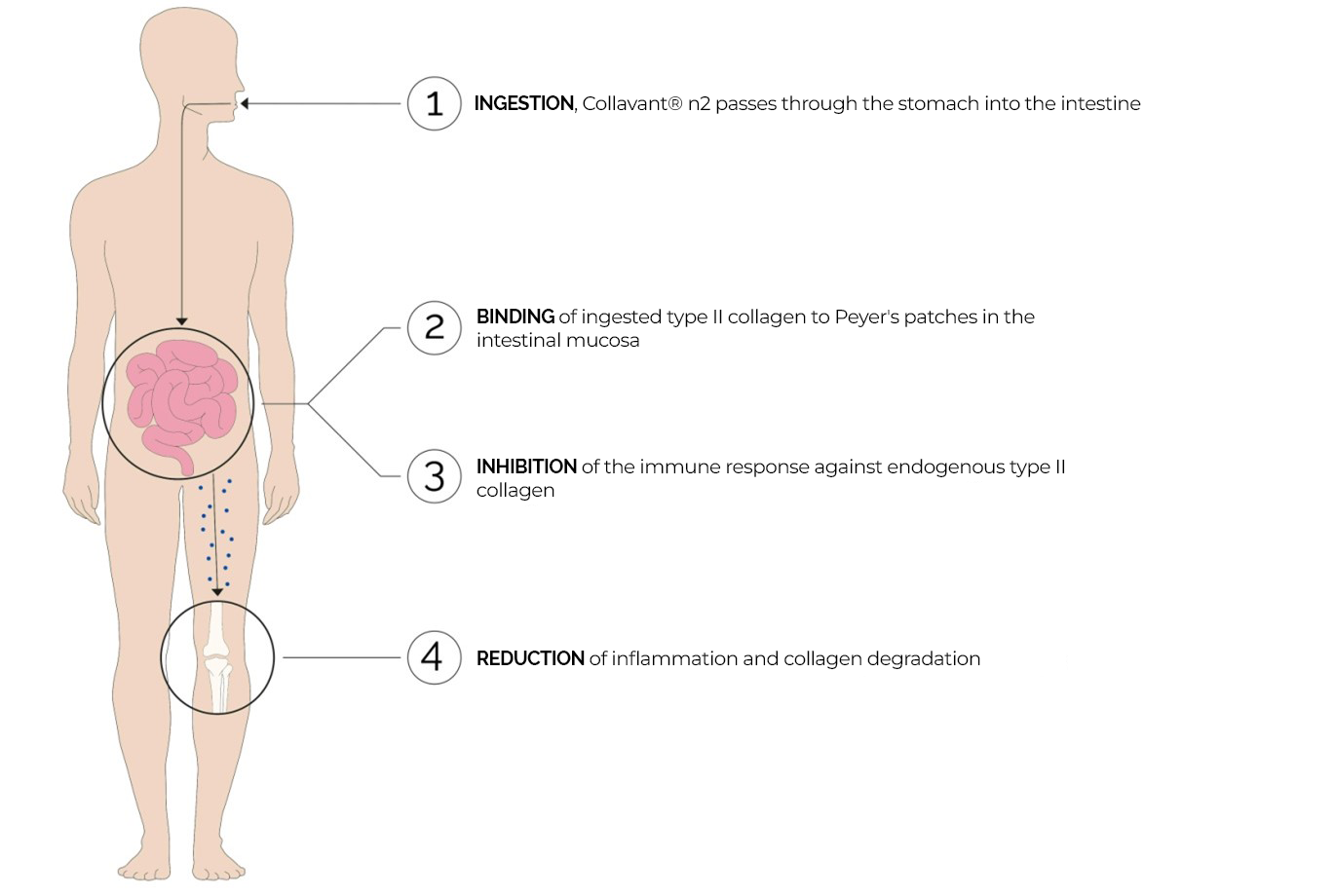
Figure 2 : Functional diagram of the mechanism of action of Collavant n2 by the oral tolerance principle
Taken orally, native collagen passes into the stomach without being denaturated (1). In the intestines, it is recognised by Peyer patches, lymphoid follicles that regulate the immune response (2). Thanks to its specific epitopes, collagen interacts with the immune cells (3), triggering the down regulation of the inflammatory process through an immune mediator. Since lymphocytes no longer act on the type II collagen present in joints (4) (Park et al., 2009), the organism then learns to recognize it as a non-harmful molecule.
The induced immune response is thus weaker, reducting collagen degradation (Manelli et al., 2015).
Native type II collagen, through this mechanism of action, reduces collagen degradation, in contrast to hydrolysed collagen which simply increases collagen synthesis.
With this mechanism of action, Collavant n2 plays a beneficial role in joint health, and its effect have been demonstrated in 3 clinical trials.
Sources
- Bakilan et al. (2016). Effects of native type II collagen treatment on knee osteoarthritis: a randomised controlled trial. Eurasian J Med, 48 (2), pg. 95-101. https://doi.org/10.5152/eurasianjmed.2015.15030
- Mannelli and al. (2015). Low dose chicken native type II collagen is active in a rat model of osteoarthritis. Osteoporosis Int., 26, pg. 184.
- Kandahari et al. (2015). Recognition of Immune Response for the Early Diagnosis and Treatment of Osteoarthritis. Journal of Immunology Research 2015, 192415. https://doi.org/10.1155/2015/192415
- Park et al. (2009). Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod Rheumatol 19, 581. https://doi.org/10.1007/s10165-009-0210-0
- Shoulders et al. (2009). Collagen structure and stability. Annu Rev Biochem, vol. 78, pg. 929- 958. https://doi.org/10.1146/annurev.biochem.77.032207.120833.
- Thiry et al. (2019). Histologie Générale, Edition DUNOD, pg. 304.
Many people are confronted with age-related issues and joint pain, due to joint tissue degradation, arising from aging, sport practice, being overweight, having a sedentary lifestyle or diseases such as osteoarthritis or rheumatoid arthritis.
Collavant n2 is an innovative solution for joint health and has been the subject of relevant clinical trials. The effectiveness of Collavant n2 inrelieving joint pain, reducing inflammation and preventing cartilage destruction has been demonstrated in a pre-clinical study on an arthritis animal model (Mannelli et al., 2015), as well as in two clinical studies in arthritis patients: one retrospective (Scarpellini et al., 2008) and the other single-blind (Bakilan et al., 2016).
1. Pre-clinical in vivo study (Mannelli et al., 2015)
Mannelli’s in vivo study (Mannelli et al., 2015) demonstrated the protective effects on cartilage of native type II collagen extracted from chicken sternum.
This study assessed the pre-clinical effectiveness of a low dose of chicken type II collagen in rats with monosodium iodoacetate (MIA)-induced arthritis. Often related to age, osteoarthritis affects the joints and is accompanied by inflammation, pain and stiffness.
The study was carried out on four groups of rats for 14 days :
- A healthy control group : no osteoarthritis was induced and a placebo was administered.
- A negative control group: MIA-induced osteoarthritis was induced on the first day of the trial and a placebo was administered.
- A treatment group that received Collavant n2 (native type II collagen) : MIA-induced osteoarthritis was induced on the first day of the trial and a 3mg/kg daily dose of Collavant n2 was administered.
- A reference group : MIA-induced osteoarthritis was induced on the first day of the trial and a 250mg/kg daily dose of pharmaceutical grade glucosamine was administered. Glucosamine is a compound typically used to treat arthritis-related joint pain.
Various tests and measurements were used to quantify the progression of arthritis in the groups of rats :
- A paw pressure test for pain evaluation. During this test, paw withdrawal response or vocalisation is recorded upon application of mechanical force to the paw. An algesimeter is used in order to quantify pain threshold.
- The « Animex » activity meter, to record motor activity, coordination and balance in rats. Each movement is quantified over a given lapse of time.
- Plasma samples, taken to assess the biochemical markers, in particular inflammatory cytokines the interleukin 1β (IL-1β) and the Tumor Necrosis Factor α (TNFα).
REDUCTION OF JOINT PAIN
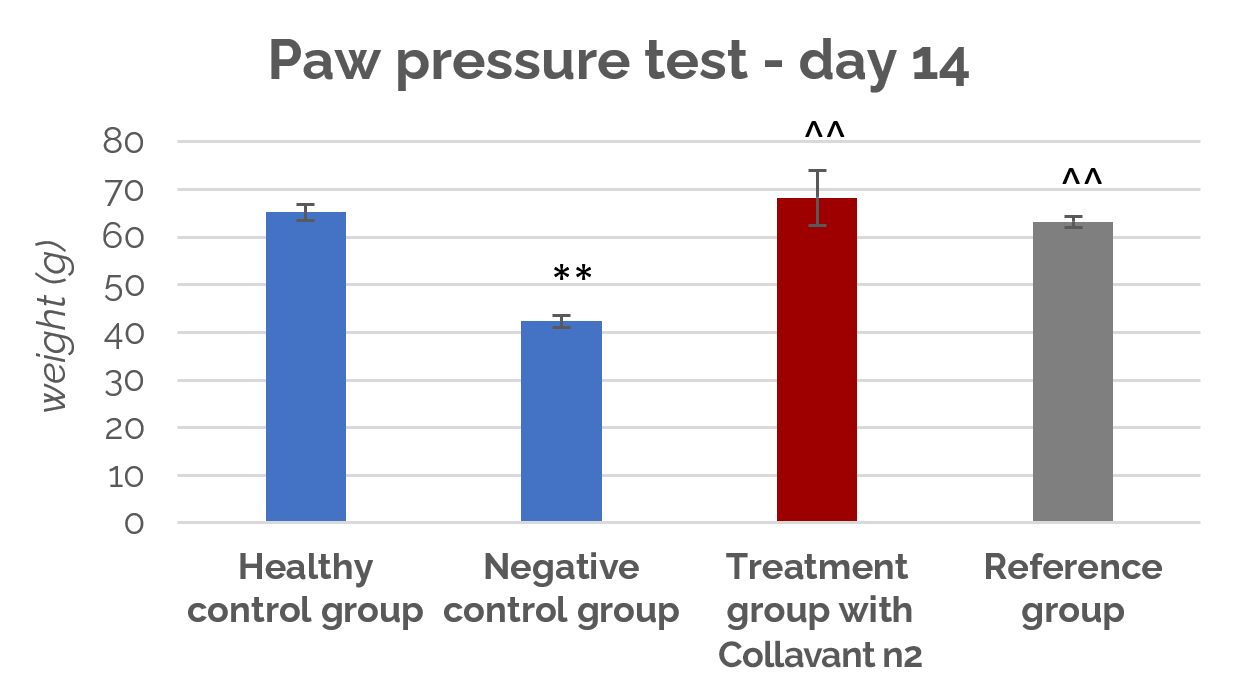
In this study, it has been shown that Collavant n2 reduced joint pain after 7 and 14 days of treatment. Indeed, the collagen-treated rats showed significantly reduced (p<0,01) sensitivity to pain upon mechanical pressure applied to the paw, compared to the negative control group.
As shown in figure 1, the pressure tolerated in the affected paw of rats treated with 3mg/kg Collavant n2 is significantly higher than in the untreated control group (p<0,01) on day 14.
Figure 1 : Tolerated pressure (g) during paw pressure test after 14 days of treatment
The « Animex » test corroborates these results and demonstrates that daily supplementation with Collavant n2 improved motor activity, coordination and balance.
Thus, at very low doses, Collavant n2 supports joint health and its effectiveness is comparable to that of pharmaceutical grade glucosamine used at much higher dose.
DECREASE OF THE INFLAMMATORY RESPONSE
The Collavant n2 treatment is not only associated with behavioural improvements but also with an improvement in biochemical markers.
Figure 2 shows that on the 14th day, Collavant n2 significantly reduced the disease-induced increase in plasma IL-1β and TNFα levels (p<0,01) with respect to the untreated group. These results were similar to the effects of glucosamine.
Collavant n2 also decreased endogenous collagen degradation, measured by serum levels of collagen fragments, exerting a protective effect on cartilage.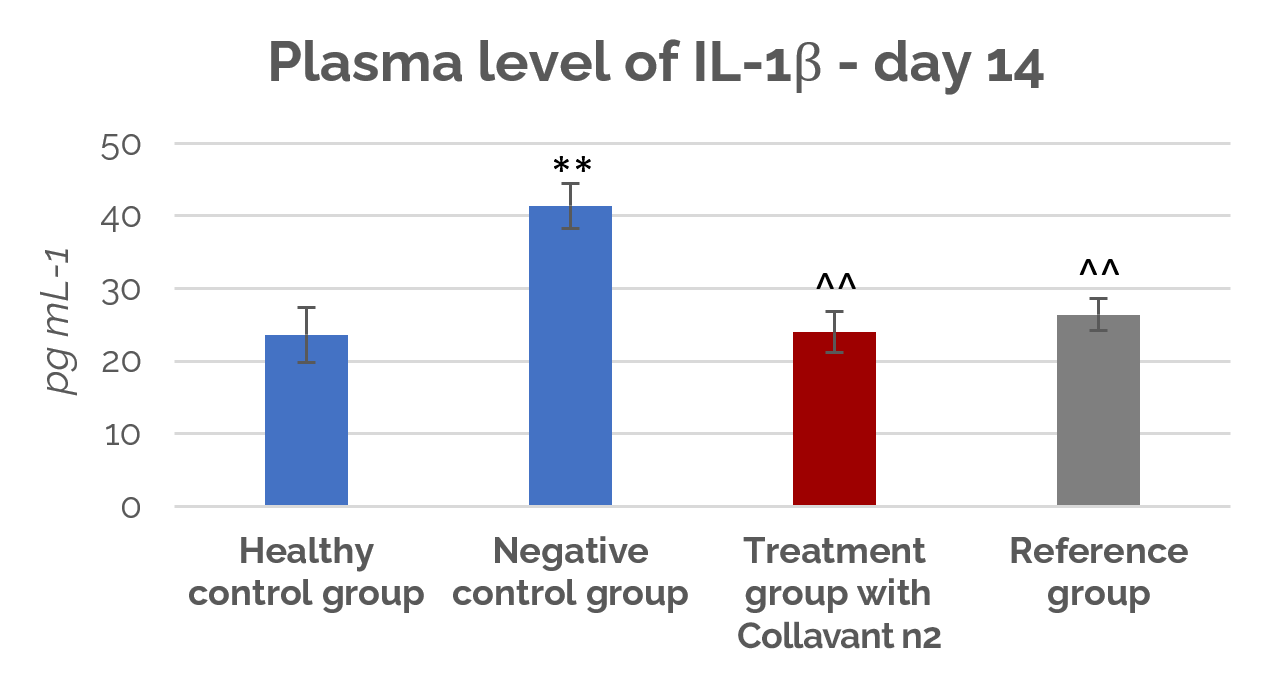
Figure 2: Plasma IL-1ß levels at day 14
In conclusion, this study helped to shed light on the positive effects of the supplementation with Collavant n2 in supporting joint health.
2. Retrospective clinical study (Scarpellini et al., 2008)
The aim of the Scarpellini et al. (2008) study was to assess the effect of Collavant n2 (native type II collagen) in combination with glucosamine and chondroitin sulphate, two ingredients known for their positive effect on joint. This comparative retrospective study followed 104 patients (average age 61.4 years) with osteoarthritis in the hips, knees, hands or erosive hand osteoarthritis (a severe form of arthritis).
They were divided into two groups :
- A control group : 47 patients were supplemented with 1000mg of glucosamine and 1000 mg of chondroitin sulfate.
- A Collavant n2 supplemented group : 57 patients were supplemented with 2mg of Collavant n2, 1000mg of glucosamine and 1000mg of chondroitin sulfate.
Various test and evaluation procedure were carried out on the patients at the beginning of the experiment (T0), after 6 months (T6) and after 12 months (T12):
- A questionnaire with a visual analogue scale (VAS) to assess resting pain and pain during walking.
- Urine test using ELISA kit CroosLapsâ et CartiLapsâ to quantify biomarkers uCTX I et uCTX-II, collagen peptides generated during cartilage degradation.
- A radiological assessment of joints at T0 and at T12 (Kellgren and Lawrence grade from 0 to 4, 0 corresponding to a normal X-ray and 4 to severe osteoarthritis).
EFFETS of GLUCOSAMINE, CHONDROITIN AND Collavant n2 ON OSTEOARTHRITIS
After 6 and 12 months, there is a decrease in pain at rest and while walking for all participants compared to baseline (pT6 = 0,014 et pT12 = 0,004).
Moreover, both treatments significantly decreased the concentration of the biomakers uCTX-I (pT6=0,002 et pT12=0,002 vs T0) and uCTX-II (pT6=0,003 et pT12=0,002 vs T0) compared to baseline for all participants.
These results showed a decrease in cartilage degradation and pain after only six months.
EFFETS OF Collavant n2 ON CARTILAGE DEGRADATION
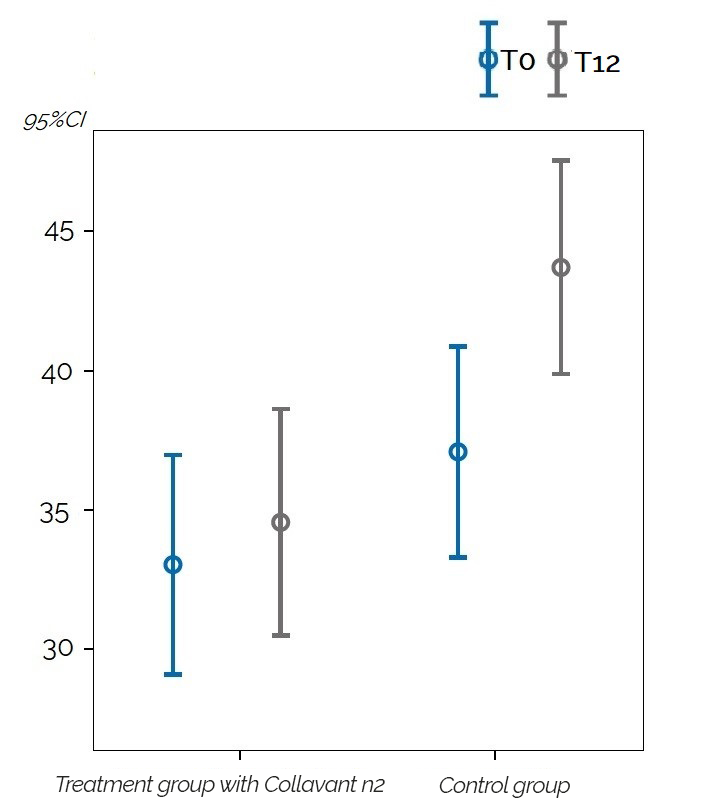
Significant results were observed in people with hand osteoarthritis or erosive hand osteoarthritis. The quantity of the uCTX-I decreased significantly after 12 months by 35% (p=0,026) for the whole study population and by 60% (p=0,017) for erosive hand osteoarthritis patients in the group that received the Collavant n2-containing treatment compared to the control group (figure 3).
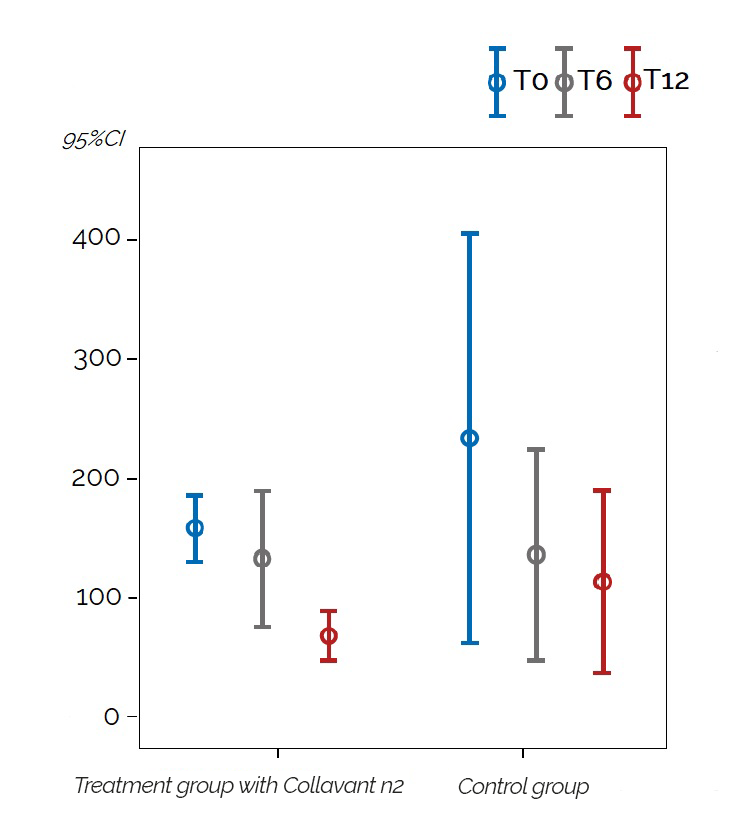
Figure 3 : Change in the mean uCTX-I concentrations in patients with erosive hand osteoarthritis overtime for the groupe that received the Collavant n2-containing supplements and the control groupe ( 95% CI: 95% confidence interval).
Moreover, erosive and non-erosive hand osteoarthritis patients in the Collavant n2-treated group showed further improvement at 12 months of treatment for the uCTX-II concentrations compared with 6 months of treatment, whereas the control group shows a tendency to regress at 12 months with respect to 6 months prior (p=0.017).
The addition of Collavant n2 decreased the collagen fragments generated during cartilage degradation (mean levels of the uCTX-I and uCTX-II markers) compared with the control group, particularly in people with hand osteoarthritis. Therefore, the combination with Collavant n2 appears to reduce the degradation of collagen more than glucosamine and chondroitin alone.
In parallel, figure 4 shows decreased progression in the Kellgren-Lawrence scale that assesses the severity of osteoarthritis. At 12 months, the Collavant n2 group shows a significantly slower increase in their Kellgren-Lawrence score (p=0.009) than the control group. Therefore, this result suggests that adding Collavant n2 helps slowdown the structural progression of osteoarthritis.
Figure 4 : Change overtime in the Kellgren-Lawrence score in erosive hand arthrisis patients for the group supplemented with Collavant n2 and the control group (95% CI: 95% confidence interval)
To conclude, the study’s results showed the effectiveness of Collavant n2 supplementation in reducing collagen degradation and slowing down osteoarthritis progression.
3. Randomised single-blind clinical study (Bakilan et al., 2016)
Bakilan et al. (2016) clinical study assessed the effect of Collavant n2 supplementation on knee osteoarthritis. This randomised single-blind controlled clinical study evaluated the efficacy of Collavant n2 during a 3 months treatment.The 39 participating patients, aged from 45 to 70 years and all suffering from knee arthritis, were divided into two groups:
- A control group, which received 1500 mg/day of paracetamol (opioid analgesic).
- A treatment group, which received 40 mg/day of Collavant n2 and 1500 mg/day of paracetamol.
Various tests and measurements were carried out on the patients in the pre-treatment (t0) and post-treatment phase (t3):
- A questionnaire with a visual analogue scale (VAS) that assesses pain at rest and during walking.
- A WOMAC questionnaire, a recognised index that measures osteoarthritis in the lower limbs. It is based on self-reported pain, stiffness and physical functioning. Physical functioning is the difficulty experienced in performing daily activities such as climbing stairs, standing, putting on socks, etc…
- A standardised questionnaire on quality of life (SF36), with 36 questions on health status on 8 scales (physical role functioning, bodily pain, general health, emotional role functioning, social role functioning, vitality, mental health)
- Urine samples using ELISA kits for the quantification of biomarkers related to cartilage breakdown (Coll 2-1, Coll 2-1NO2 and fibulin-3).
RESULTS
After 3 months of treatment, patients in the Collavant n2 supplemented group reported significant improvements compared to t0 regarding pain, function and quality of life:
- VAS shows a reduction in pain during walking (p<0.001).
- WOMAC shows an overall improvement in joint function (WOMAC total score; p=0.004). More specifically, there is a reduction in pain (p=0.003) and an improvement in physical function (p=0.016).
- The SF36 shows significant improvements for several sub-categories of the questionnaire such as physical functioning (p=0.039), physical role (p=0.023), emotional role (p=0.048) and body pain (p=0.016).
At the end of the 3 months of treatment, there was a significant difference in the VAS walking score in favour of the Collavant n2 supplemented group compared to the control group (p=0,014). A 50% reduction in pain was recorded in the group supplemented with Collavant n2 and paracetamol (p=0.002).
To conclude, supplementation with native type II collagen (Collavant n2) combined with an analgesic would therefore reduce joint pain, improve mobility and quality of life after 3 months of treatment in people with knee osteoarthritis. Moreover, Collavant n2 supplementation would have a superior effect to paracetamol alone in reducing joint pain during walking.
Sources
- Mannelli et al. (2015). Low dose chicken native type II collagen is active in a rat model of osteoarthritis. Osteoporosis Int, volume 26, pg. 366.
- Scarpellini et al. (2008). Biomarkers, type II collagen, glucosamine and chondroitin sulfate in osteoarthritis follow-up: the “Magenta osteoarthritis study”. Journal of orthopaedics and traumatology, volume 9 (2), pg. 81–87. https://doi.org/10.1007/s10195-008-0007-5
- Bakilan et al. (2016). Effects of Native Type II Collagen Treatment on Knee Osteoarthritis: A Randomized Controlled Trial. The Eurasian journal of medicine, 48(2), pg. 95-101. https://doi.org/10.5152/eurasianjmed.2015.15030
GENERAL INFORMATION:
Collagen is a general use food ingredient included in Annex III, Section XV of Regulation n°853/2004 laying down specific hygiene rules for food of animal origin.
As there is history of collagen use in foods prior to May 15th, 1997, Regulation (UE) n°2015/2283 regarding novel foods does not apply. Thus, Collavant n2 can be used as an ingredient added to foods or included in food supplements formulations.
Collagen is authorised in France in food supplements by mutual recognition (Article 16).

Collavant n2 is an ingredient that undergoes a thorough process control at Bioiberica. Once extracted from the chicken sternum, the product follows a strictly controlled manufacturing process to obtain a fine, cream-coloured, odourless powder.
Collavant n2 is mainly used in the formulation of food supplements in powder, tablet and capsule form.
According to the studies conducted by Bioiberica, the recommended daily dose is 40 mg. This dose is 250 times lower than the amount typically used for hydrolysed collagen (10 g/day). This considerably lower dose greatly facilitates formulation in compact formats.
Collavant n2 can be associated with vitamin C, vitamin D and certain minerals (copper, manganese, zinc, magnesium) ensuring the good functioning of mechanisms underlying joint functioning and/or participating in the formation of collagen.