
Pylopass™- Lactobacillus reuteri
Gastrointestinal health
Pylopass™ is a patented strain of Lactobacillus reuteri that offers a new approach to the control of Helicobacter pylori: primary risk factor for ulcers and gastritis.
Pylopass™ has a unique mode of action; Lactobacillus reuteri coaggregates specifically with Helicobacter pylori and so reduces its bacterial load in the stomach.
Pylopass™ is available in France and Benelux.
=> This content is intended exclusively for professionals (food supplements laboratory and manufacturer)
Pylopass™ is a culture of Lactobacillus reuteri (DSM 17648) containing ≥ 1 x10^11 inactivated cells/gram.
This is an ingredient for digestive health making it possible to control Helicobacter pylori in the stomach.
Pylopass™ was selected from among 700 wild-type strains of Lactobacillus species for its ability to act as a Helicobacter pylori antagonist.
1. Mode of action
Known by the scientific community, Lactobacillus reuteri is a lactic acid bacterium belonging to the native bacteria in man and animals. It is a rod-shaped, non-spore-forming, Gram-positive bacterium.
Helicobacter pylori is a helix-shaped Gram-negative bacterium that colonises the stomach. This bacterium is now known as the major cause of ulcers and gastritis. It is thought that 50% of the world’s population is infected by this bacterium, more prevalent in developing countries where up to 90% are infected (Go MF, 2002).
According to Kusters et al. (2006), the severity of symptoms is linked to the bacterial load and an untreated ulcer or gastritis can lead to cancer.
Thus, Pylopass™ is a very interesting solution against H. pylori, thanks to a unique mode of action.
Lactobacillus reuteri binds specifically to H. pylori in the stomach. The adhesion molecules at the surface of Pylopass™ recognise and adhere to the surface of Helicobacter pylori. Aggregates form in the stomach are then excreted through the digestive tract and eliminated from the body (Figure 1).
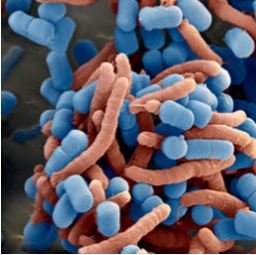
Figure 1 : Coaggregation of Pylopass™ (coloured blue) and H. pylori (coloured red)
(Scanning electron microscopy, magnification 11,000x) (Holz C. et al., 2014).
2. Fermentation process
Pylopass™ is obtained by a fermentation process and drying (atomisation) from cells of Lactobacillus reuteri. This is done at low temperature to avoid denaturing the adhesion molecules at the surface of the cells. The fermentation is standardised to coaggregation levels. This means that Pylopass™ products have been fermented to ther peak aggregation abilities.
The Lactobacillus reuteri cells are inactivated during the process. This means that Pylopass™ is stable under the gastric conditions. Its mechanism of action does not actually depend on its survival in the stomach and it doesn’t actually depend on its survival in the stomach and it doesn’t disturb the gut microbial balance.
Sources
- Go MF (2002). Review article: Natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther 16: 3–15.
- Kusters JG, van Vliet AHM, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19:449–490.
- Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ, Kuipers EJ (2012) Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut 61:646–664 7.
- Wu TS, Hu HM, Kuo FC, Kuo CH (2014) Eradication of Helicobacter pylori infection. Kaohsiung J Med Sci 30:167–172.
- Holz C. et al. (2014). Significant Reduction in Helicobacter pylori Load in Humans with Non-viable Lactobacillus reuteri DSMZ17648: A Pilot Study. Probiotics & Antimicro. Prot.
Various studies have been done on the mechanism of action and health benefits of Pylopass™.
The mechanism of action of Pylopass™ reduces colonisation of the stomach by H. pylori, primary risk factor for ulcers and gastritis.
Its role is supported by six studies. The major results of the study by Mehling & Busjahn in 2013 and by Holz et al. in 2014 (in vitro and pilot study) will be introduced here.
1. In vitro results
Firstly, the in vitro study by Holz et al. assessed the effectiveness of Lactobacillus reuteri DSM 17648, the Pylopass™ strain.
The coaggregation of Lactobacillus reuteri DSM 17648 with the H. pylori DSM 21031 strain has been observed using fluorescence microscopy (figure 1 (a)). The second coaggregation of these strains has also been observed in a medium reproducing gastric conditions (pH = 4, artificial gastric juice, 0.3% pepsin, 0.5% sodium chloride) (figure 1 (b)).
The coaggregation of the two bacteria is clearly highlighted under the microscope in figure 1 (a – B). In figure 1 (b), the coaggregation of the two bacteria can also be seen with the naked eye; a change is visible in beaker B although a change is not observed in culture media containing either H. pylori or Lactobacillus reuteri. (A and C)
The first in vitro results highlight the specific mechanism of Pylopass™.
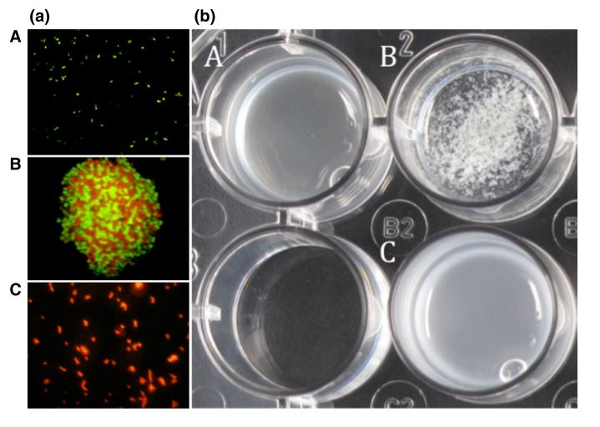
Figure 1 : Microscopic (a) and macroscopic (b) examination of the coaggregation of Lactobacillus reuteri and Helicobacter pylori.
Legend:
(a) (A) H. pylori stained with hexidium iodide
(B) Coaggregation of H. pylori and L. reuteri
(C) Lactobacillus reuteri stained with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE)
(b) (A) H. pylori
(B) Coaggregation of H. pylori and Pylopass™
(C) Pylopass™
2. Pilot study results
The subsequent clinical study by Mehling & Busjahn published in 2013 evaluated the effect of Pylopass™ supplementation on Helicobacter pylori. This was a single-blind, active-controlled, crossover study on 22 H.pylori-contaminated and asymptomatic participants with average age 47 years. The subjects received a placebo for 14 days then Pylopass™, 200 mg/day for a further 14 days. The doses of 200 mg/day were administered as two 250 mg tablets taken with breakfast and two with dinner.
UBT test
Bacterial infection with Helicobacter pylori is measured using a breath test with urea labelled with carbon-13 (Urea Breath Test – UBT). This non-invasive method is based on the ability of Helicobacter pylori to synthesise urease, responsible for hydrolysing urea. (figure 2) Thus, Helicobacter pylori converts urea, labelled with carbon-13, into carbon dioxide and ammonia.
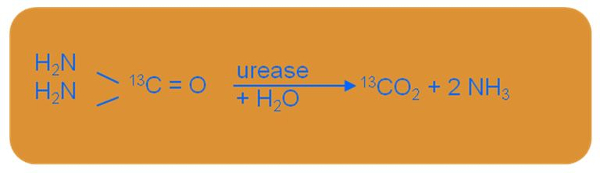
Figure 2: Hydrolysis reaction of urea caused by Helicobacter pylori (Organobalance, 2016).
A solution of labelled urea (75 mg) is swallowed by the patient, then a certain amount of isotope-labelled carbon dioxide is measured in exhaled breath. The exhaled breath sample is measured before swallowing the urea (T 0) and 30 minutes (T 30) later using mass spectrophotometry, giving a value known as the UBT value.
The test was performed at the beginning of the study, after 14 days’ supplementation with placebo and after 14 days’ supplementation with Pylopass™.
The test is positive if the 13C/12C ratio (12 C: exhaled natural carbon) increases by 4‰ after thirty minutes. This means that the individual is infected with Helicobacter pylori.
Labenz et al. (1993) and Zagari et al. (2005) showed that there is a quantitative relationship between urease activity and the quantity of 13C in fasting patients and therefore an indirect relationship with the degree of colonisation by H. pylori. In this study, a moderately high degree of colonisation is defined if the UBT value is greater than 12‰. Participants were selected at the start of the test by using this value (UBT > 12). A Wilcoxon non-parametric statistical test was applied to compare the effect of the placebo and Pylopass™.
Figure 3 shows the relative variation of the value after 14 days of Placebo (in blue) and the value obtained after 14 days of Pylopass™ (in brown) compared to baseline values.
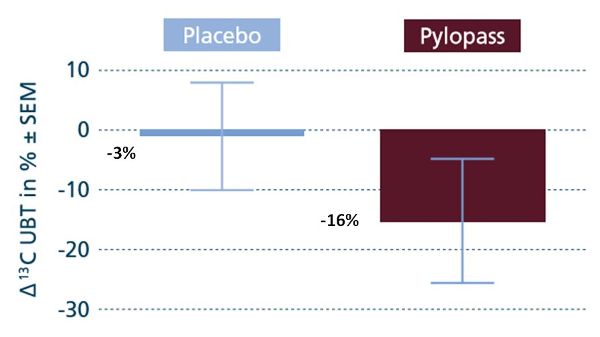
Figure 3: Comparison between placebo and Pylopass™.
There was a significant reduction of – 16% (16‰ vs 20‰, p < 0.05) in the UBT test value after 14 days’ supplementation with Pylopass™. The reduction of – 3% with the placebo was not significant.
Holz et al. (2014) also studied the effect of two weeks’ supplementation with Pylopass™ on the bacterial load of Helicobacter pylori in the stomach. This was a single-blind, randomised, active-controlled cross over pilot study involving 24 participants.
At the start, the participants were contaminated by Helicobacter pylori but did not yet have symptoms.
They received two tablets of 5×109 freeze-dried inactivated Lactobacillus reuteri cells (equivalent to 200 mg of Pylopass™) to be swallowed after breakfast and dinner.
The bacterial load of H. pylori was measured using a UBT test at the beginning of the study (baseline value) and after two weeks.
After 14 days’ supplementation with Pylopass™, there was a significant reduction in the UBT test value (–4.9 ± 7.8, p = 0.026 vs. placebo) compared to the placebo group (–0.6 ± 5.3) and therefore a reduction in the contamination by H. pylori.
Pylopass™ therefore significantly reduces the bacterial load of Helicobacter pylori in the stomach and therefore a reduction in the risks of ulcers and gastritis.
For more information about Pylopass™:
Sources
- Mehling H et al (2013). Non-Viable Lactobacillus reuteri DSMZ 17648 (Pylopass™) as a New Approach to Helicobacter pylori Control in Humans. Nutrients 5, 3062-3073
- Holz C. et al (2014). Significant Reduction in Helicobacter pylori Load in Humans with Non-viable Lactobacillus reuteri DSM17648: A Pilot Study. Probiotics & Antimicro. Prot.
The strain of Pylopass™ is part of the Novozymes Berlin GmbH, formerly called Organobalance
Pylopass™ was selected for its quality as a Helicobacter pylori antagonist.
Status
Pylopass™ belongs to the list of microorganisms that have obtained QPS status (Qualified Presumption of Safety) defined by the EFSA in Europe (2007) by virtue of its safe use in food (1).
It has GRAS status in the United States.
A patented strain
It has been lodged at the Leibniz Institute, German microorganism and cell culture bank, under number DSM 17648.
The use of Lactobacillus reuteri (DSM 17648.) against Helicobacter pylori is protected in Europe by patent EP1963483 B1.
A second patent EP2717890 B1 also protects the use of Lactobacillus reuteri dried by atomisation against H. pylori.
Additional information
Pylopass™ is a non-allergenic strain (1), non-GMO (2) , gluten-free (3), halal and suitable for vegetarians.
Sources
- (1) Annex II of Regulation (EU) No 1169/2011
- (2) Regulations n°1829/2003/EC, 1830/2003/EC
- (3) Codex Stan 118-197
Pylopass™ can be used to formulate food supplements; soft or hard capsules, tablets, sachets, etc. It can also be used as powder in drinks.
The recommended daily dose, defined from clinical studies, is 200 mg. This quantity can be split into different doses.
1 g of Pylopass™ is equivalent to approximately 100 billion cells of Lactobacillus reuteri.
Pylopass™ is a very stable ingredient not requiring refrigeration as, unlike probiotics, these inactivated strains tolerate changes in environmental conditions perfectly.
Please leave us your contact information to see the webinar replay on Pylopass™:
